Focused On-demand Libraries - Receptor.AI Collaboration
Explore the Potential with AI-Driven Innovation
This extensive focused library is tailor-made using the latest virtual screening and parameter assessment technology, operated by the Receptor.AI drug discovery platform. This technique is more effective than traditional methods, offering compounds with improved activity, selectivity, and safety.
The compounds are cherry-picked from the vast virtual chemical space of over 60B molecules. The synthesis and delivery of compounds is facilitated by Reaxense.
Contained in the library are leading modulators, each labelled with 38 ADME-Tox and 32 physicochemical and drug-likeness qualities. In addition, each compound is illustrated with its optimal docking poses, affinity scores, and activity scores, giving a complete picture.
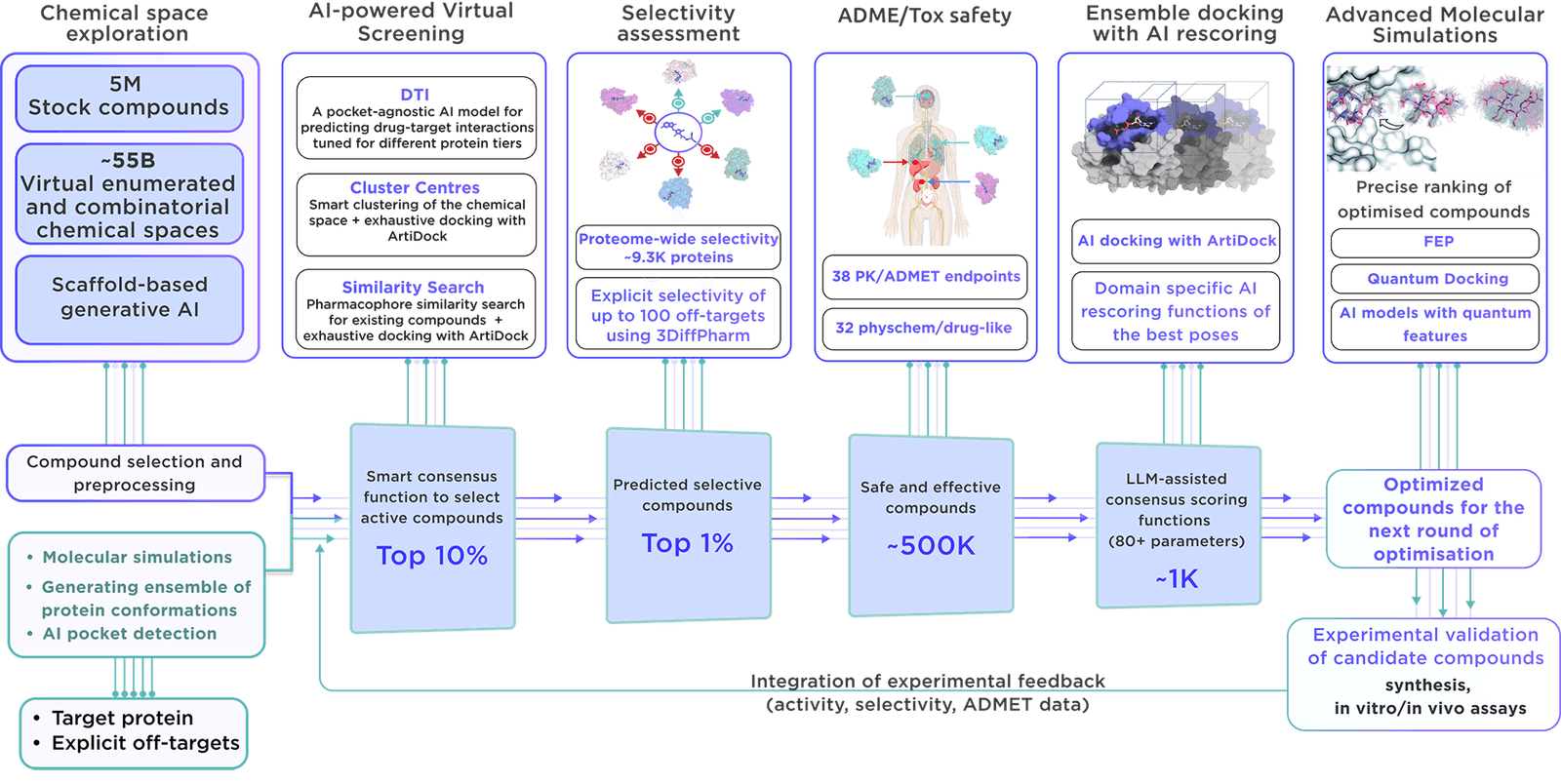
We use our state-of-the-art dedicated workflow for designing focused libraries.
Our methodology leverages molecular simulations to examine a vast array of proteins, capturing their dynamics in both isolated forms and in complexes with other proteins. Through ensemble virtual screening, we thoroughly account for the protein's conformational mobility, identifying critical binding sites within functional regions and distant allosteric locations. This detailed exploration ensures that we comprehensively assess every possible mechanism of action, with the objective of identifying novel therapeutic targets and lead compounds that span a wide spectrum of biological functions.
Several key aspects differentiate our library:
- Receptor.AI compiles an all-encompassing dataset on the target protein, including historical experiments, literature data, known ligands, and structural insights, maximising the chances of prioritising the most pertinent compounds.
- The platform employs state-of-the-art molecular simulations to identify potential binding sites, ensuring the focused library is primed for discovering allosteric inhibitors and binders of concealed pockets.
- Over 50 customisable AI models, thoroughly evaluated in various drug discovery endeavours and research projects, make Receptor.AI both efficient and accurate. This technology is integral to the development of our focused libraries.
- In addition to generating focused libraries, Receptor.AI offers a full range of services and solutions for every step of preclinical drug discovery, with a pricing model based on success, thereby reducing risk and promoting joint project success.
Receptor.AI
P82673
UPID:
RT35_HUMAN
ALTERNATIVE NAMES:
28S ribosomal protein S28, mitochondrial; 28S ribosomal protein S35, mitochondrial
ALTERNATIVE UPACC:
P82673; Q32LZ1; Q6P4C6; Q7L1M6; Q8NBP4; Q96AI0; Q9H044; Q9HC14; Q9P1R5