Research Background
Protein kinases is a numerous group (over 500 members) of phosphate transfering enzymes playing crucial role in signal transduction of the living cell. These regulatory enzymes control most of the functions in metabolism, growth and differentiation of various cell types. Changes in the level, subcellular location and activity of kinases have consequences on normal cell function and maintenance of cellular homeostasis that leads to the onset of several diseases including cancer, immunological, neurological, metabolic and infectious disease. This has created a plethora of potential targets that can be studied in a unified way, but the field faces significant challenges from drug resistance, lack of inhibitor selectivity, lack of inhibitor efficacy and difficulty in drug target validation for particular disease settings.
About 10% kinase domains lack residues thought to be essential for nucleotide binding and/or phosphoryl transfer activity in catalytically active kinase domains. These domains were considered to have no signalling function and termed “pseudokinase” domains. Many have been shown experimentally to lack activity, while others have been associated with weak kinase activity, with varying degrees of confidence. It has emerged that these diverse domains are essential modulators of signal transduction in bacteria, plants, toxoplasma parasitic invasion, and in vertebrates. It has been proven that some pseudokinases have catalytic activity (or possess an intact ATP-binding site and retain the ability to bind to ATP) while others serve as scaffold proteins in multienzyme complexes, or perform allosteric regulation (activation) of other, “true” enzymes. Aberrant regulation of pseudokinases is implicated in the cause and development of a variety of diseases, particularly in malignancies, that makes this type of proteins an attractive and unexplored class of drug targets. Designing drugs to block pseudokinases is a challenge because the traditional and well-studied enzyme active site could not be the natural place to hit and researchers have to think differently about how to inhibit them. An advantage of targeting pseudo-kinases is that the structures of their ATP binding sites are likely to be quite different from those of catalytically active protein kinases, suggesting that rather selective molecules could be developed.
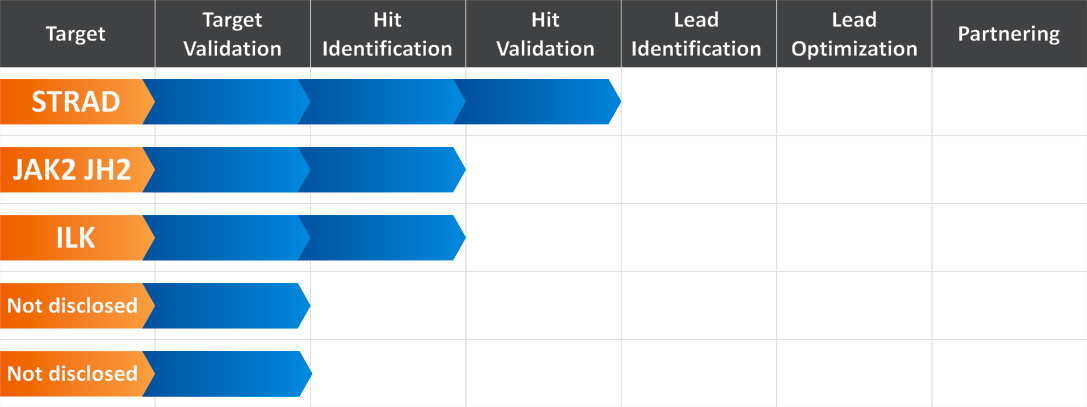
e-BioModular™ Pseudokinases Research Platform at Reaxense Inc.
Reaxense’s current drug discovery program named e-BioModular™ Pseudokinases Research Platform is aimed at the development of hit/lead compounds against a number of pseudokinase targets.
Approaches raised from ligand- and structure-based drug design concepts are being intensively used in terms of this program. After in vitro and in vivo evaluation of compounds, internal synthetic capabilities are utilized for structural optimization of the most promising lead compounds.
Contact us for more information.